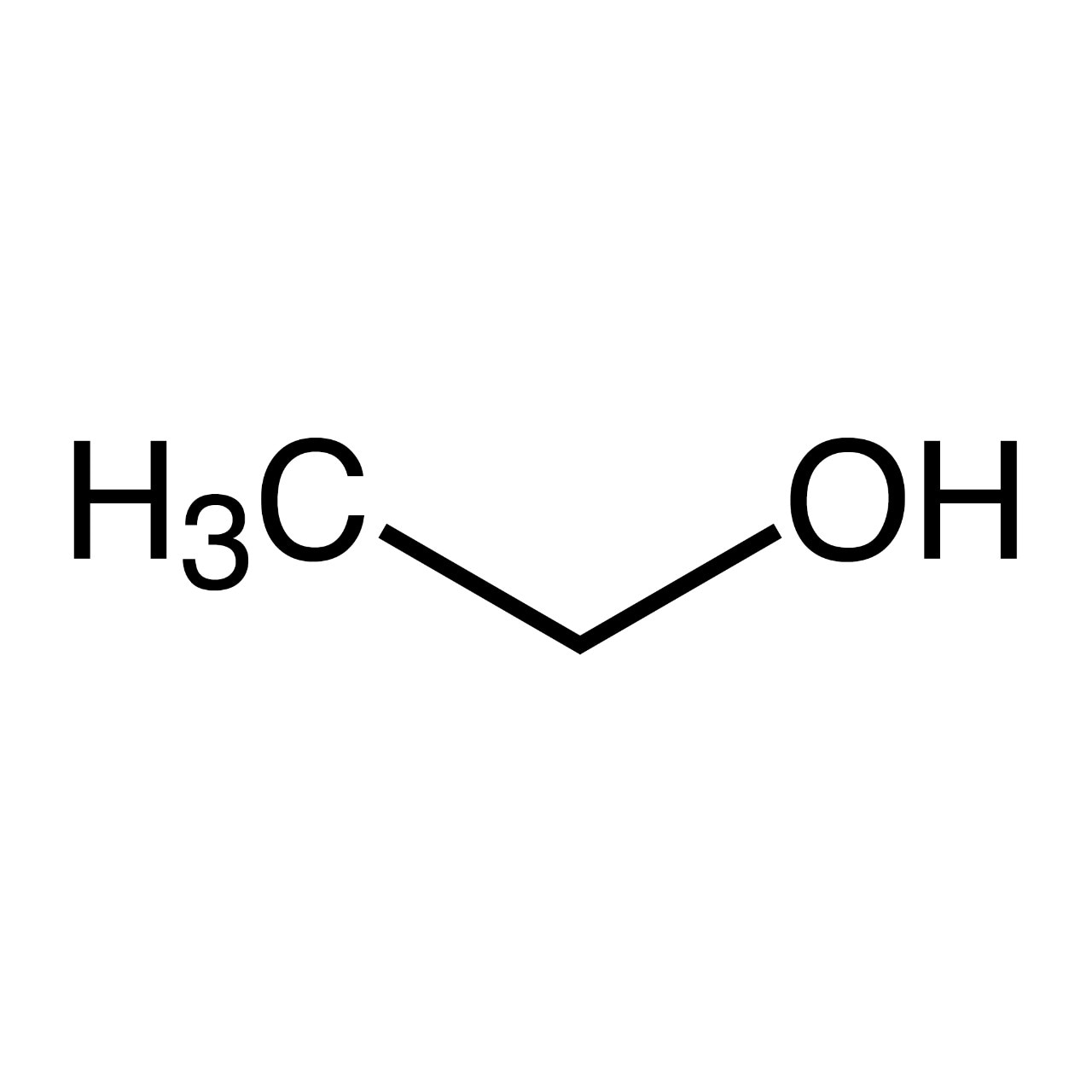
Ethanol (C2H5OH, EtOH) Fisher Scientific
Estructuras de Lewis. También utilizamos los símbolos de Lewis para indicar la formación de enlaces covalentes, que se muestran en las estructuras de Lewis, dibujos que describen el enlace en moléculas e iones poliatómicos.Por ejemplo, cuando dos átomos de cloro forman una molécula de cloro, comparten un par de electrones:
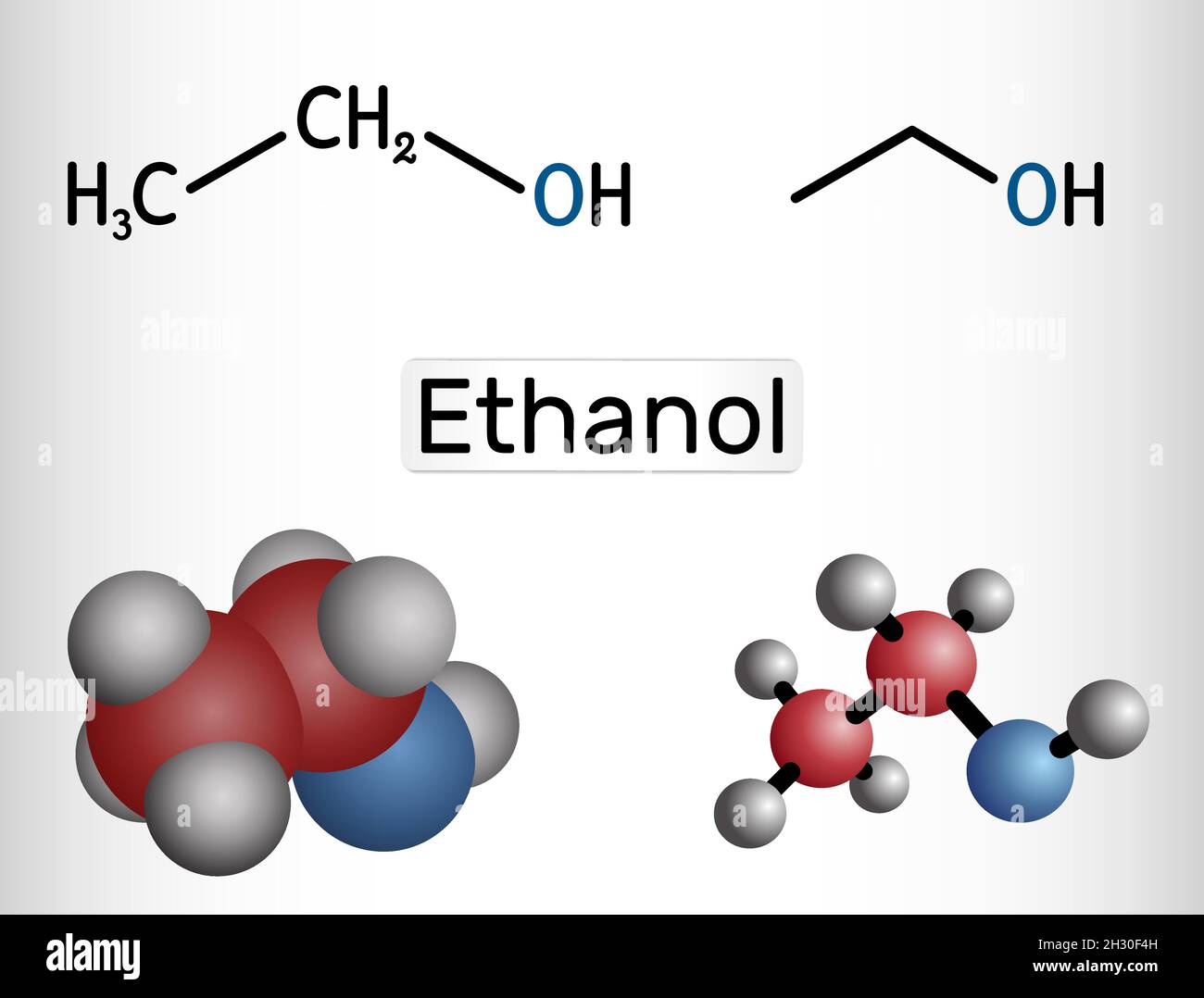
C2h5oh Lewis Structure 3d
1. Write the condensed formula for each Lewis structure. A. B. 2. Draw a line-angle structure for the compound CH 3 CH 2 CH(CH 3)CH 2 CH 2 CH 3. 3. Give the condensed formula for the compound represented by this line-angle structure: 4. Draw the Lewis structure of the molecule below, showing all atoms and all valence electrons (bonds and lone.

C2h5oh Lewis Dot Structure
C2H5OH or Ethanol can simply be called or termed alcohol and it is an organic chemical compound. The compound can also be represented as CH3-CH2-OH. Ethanol is a colorless liquid with a distinct odor and a pungent taste. It has flammable properties; when burnt, the compound gives a blue color flame. Here are some ways in which ethanol is prepared:
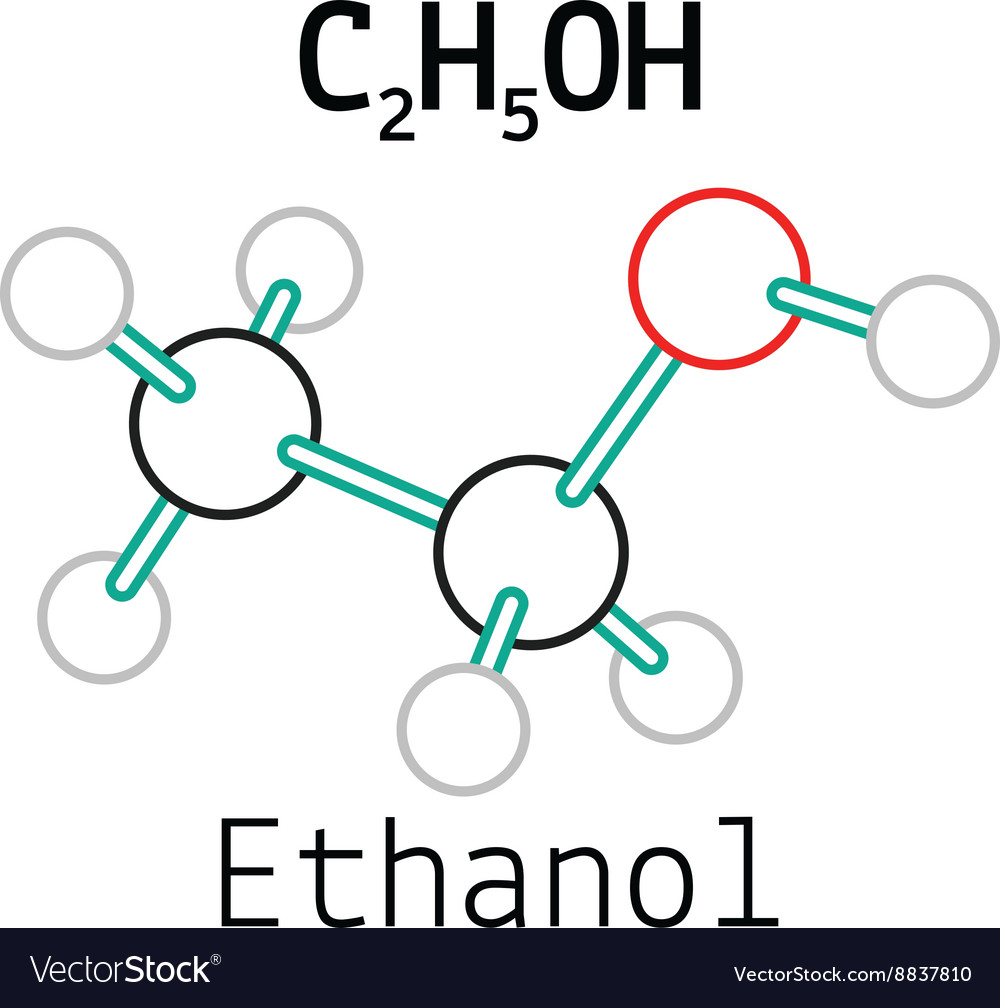
C2H5Oh Lewis Structure
Las estructuras de Lewis, también conocidas como diagramas de puntos de Lewis, muestran la relación de unión entre los átomos de una molécula y los pares solitarios de electrones en una molécula. Si bien inicialmente puede ser útil escribir los electrones compartidos individuales, este enfoque rápidamente se vuelve incómodo.

هيكل سيو 2 لويس
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
Molécula Etanol C2h5oh. Es Un Alcohol Primario Y Alcalino. Fórmula Química Estructural Y Modelo
Draw Lewis structures depicting the bonding in simple molecules. Compute formal charges for atoms in any Lewis structure. Use formal charges to identify the most reasonable Lewis structure for a given molecule. Identify the oxidation states of atoms in Lewis structures. 3.3.0: Bond Types. 3.3.0.0: Bond Types (Problems) 3.3.1: Lewis Dot Diagrams.

C2h5oh Lewis Dot Structure
Lewis Structure of C2H5OH (Ethanol): Explained. - 2 C atoms (4 e/atom) + 6 H atoms (1 e/atom) + 1 O atom (6 e/atom) = 20 total electrons. - Count valence electrons for each element based on their periodic group. - Carbon has the lowest electronegativity and forms the central atom. - Carbon sits in the center, connected to all other atoms.

C2H5OH Lewis Structure (Ethanol) YouTube
Hello Guys!In this video, we will determine the Lewis Structure of Ethanol. It has a chemical formula of C2H5OH. To find out its Lewis Structure, we first ca.
Lewis Structure For C2h5oh
Ejemplos: Aquí tomaremos la molécula de CO 2 como ejemplo para explicar el procedimiento paso a paso:. 1. Número total de electrones de valencia: 4 (átomo de C) + 2×6 (2 átomos de O) = 16. Siempre DOBLE COMPROBACIÓN: En la estructura correcta de Lewis, el número total de electrones involucrados (unión más electrones no enlazantes) debe ser igual a este número, ¡menos o más son.
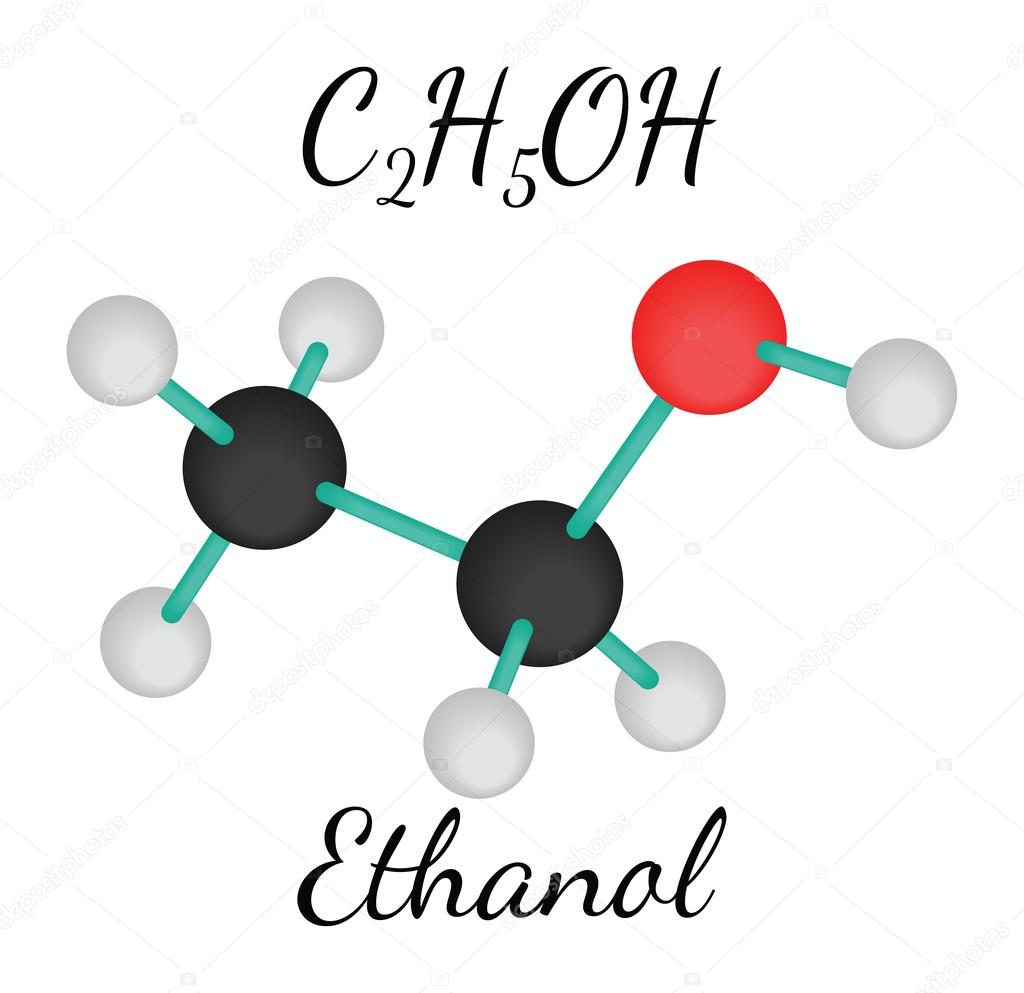
C2H5OH molécula de etanol Vector de Stock de ©MariaShmitt 100286840
Watch on. Transcript: So there are two ways presented here to draw the C2H6O Lewis structure. On the left, we have the Oxygen atom between the two Carbons. This is called dimethyl ether. On the right, the Oxygen atom's on the outside with the Hydrogen attached to it. That's called ethanol. So they're both valid Lewis structures.

C2h5oh Lewis Dot Structure
A plot of the potential energy of the system as a function of the internuclear distance (Figure 5.3.2 ) shows that the system becomes more stable (the energy of the system decreases) as two hydrogen atoms move toward each other from r = ∞, until the energy reaches a minimum at r = r 0 (the observed internuclear distance in H 2 is 74 pm).Thus at intermediate distances, proton-electron.

C2H5OH Lewis Structure, Molecular Geometry, Hybridization, and Polarity Techiescientist
A step-by-step explanation of how to draw the C2H5OH Lewis Dot Structure (Ethanol (Ethyl alcohol)).For the C2H5OH structure use the periodic table to find th.
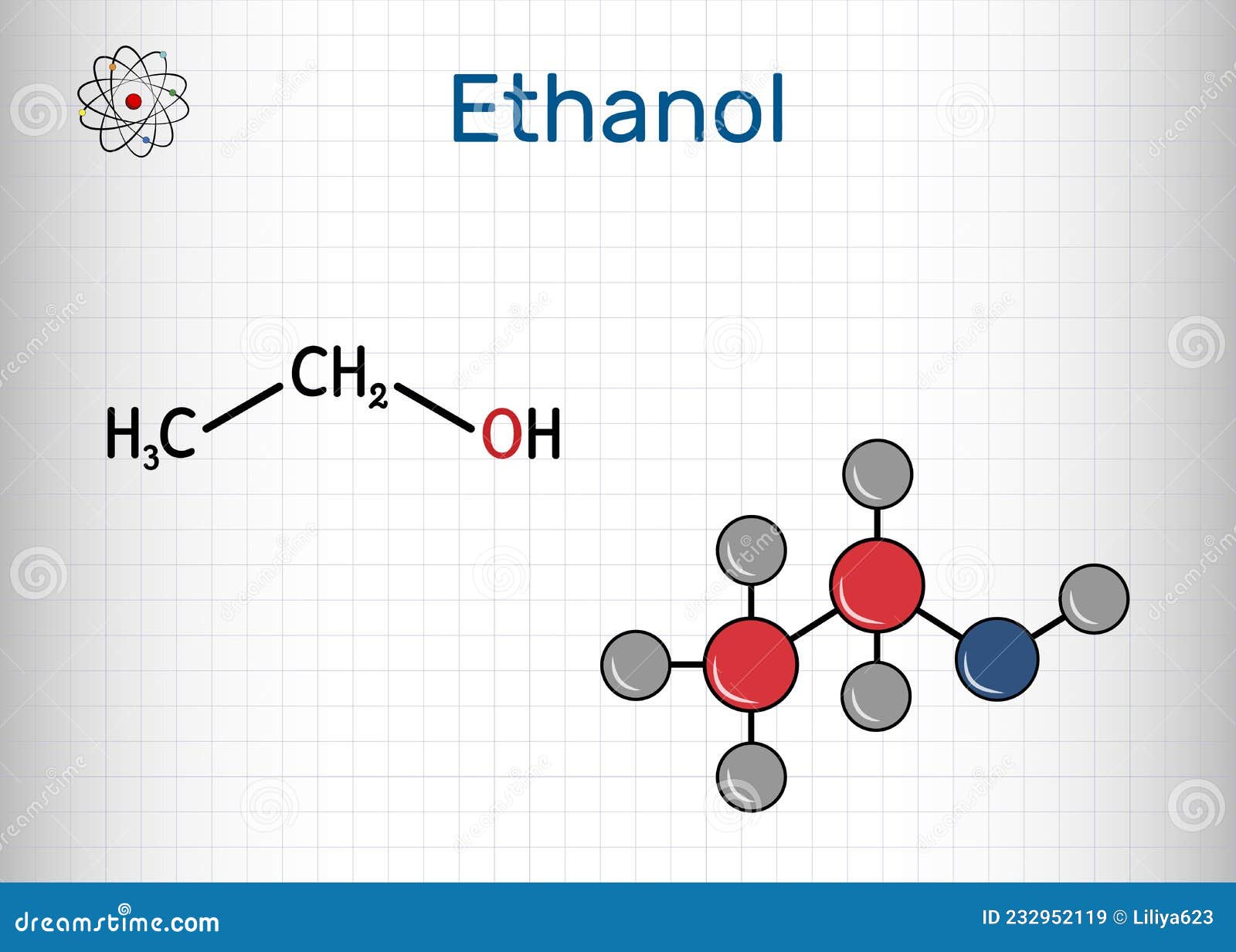
Molécula Etanol C2h5oh. Es Un Alcohol Primario Y Alcalino. Fórmula Química Estructural Y Modelo
Estructura de puntos de etanol Lewis: dibujo y explicaciones detalladas. El etanol es un compuesto químico que se utiliza comúnmente como disolvente, combustible y en la producción de bebidas alcohólicas. Su fórmula molecular es C2H5OH y consta de dos átomos de carbono, seis átomos de hidrógeno y un átomo de oxígeno.

escriba estructuras de Lewis para las siguientes moléculas que tienen solamente enlaces
C2H5OH Lewis Structure, Molecular Geometry, Bond Angles and Hybridization. C2H5OH or Ethanol is an organic chemical compound, which can also be represented as CH3-CH2-OH. Ethanol is a colourless liquid with a distinct odour and has a pungent taste. It has flammable properties; and gives a blue colour flame when burnt.

Qué es la estructura de Lewis Artículos de Arquitectura
For each of the following compounds and ions, 1. Draw a Lewis structure. 2. Show the kinds of orbitals that overlap to form each bond. 3. Give approximate bond angles around each atom except hydrogen. d. CH3CH=CH2 e. HC≡CCHO f.

2015 Respuesta libre AP Química 2 d e Química Khan Academy en Español YouTube
Dimethyl ether, also known as methoxymethane, is a colorless gas-bearing a faint odor. It has a molar mass of 46.07 g/mol and a density of 2.1146 kg/m3 as a gas at 00 C. In liquid form, it is volatile and poisonous. CH3OCH3 has its use as propellants in aerosol products like hair sprays.
- Novelas Parecidas A Betty La Fea
- Que Importancia Tiene La Arqueologia Submarina
- Que Es La Conspiracion De Queretaro
- Marvin Gaye Dream Of A Lifetime
- Que Es La Discriminacion Por Edad
- Corazon Delator Edgar Allan Poe Cuento
- La Gata Capitulo 59 Completo En Español
- Como Usar Botas Debajo De La Rodilla
- En Que Consiste La Irritabilidad
- Formas De Saludar Con La Mano